If you are interested in participating in the BPH Global Registry project, please click this link to submit your institution and we will contact you as soon as possible.
Already signed up? Follow this link to navigate to the BPH Registry login page:
In the links below, you will find resources for the principal investigators, and the participating physicians:
If you are logging in for the first time, the site will ask you to change your password. Save your username and password in your password manager, for easier recall.
Please include country code in the telephone number.
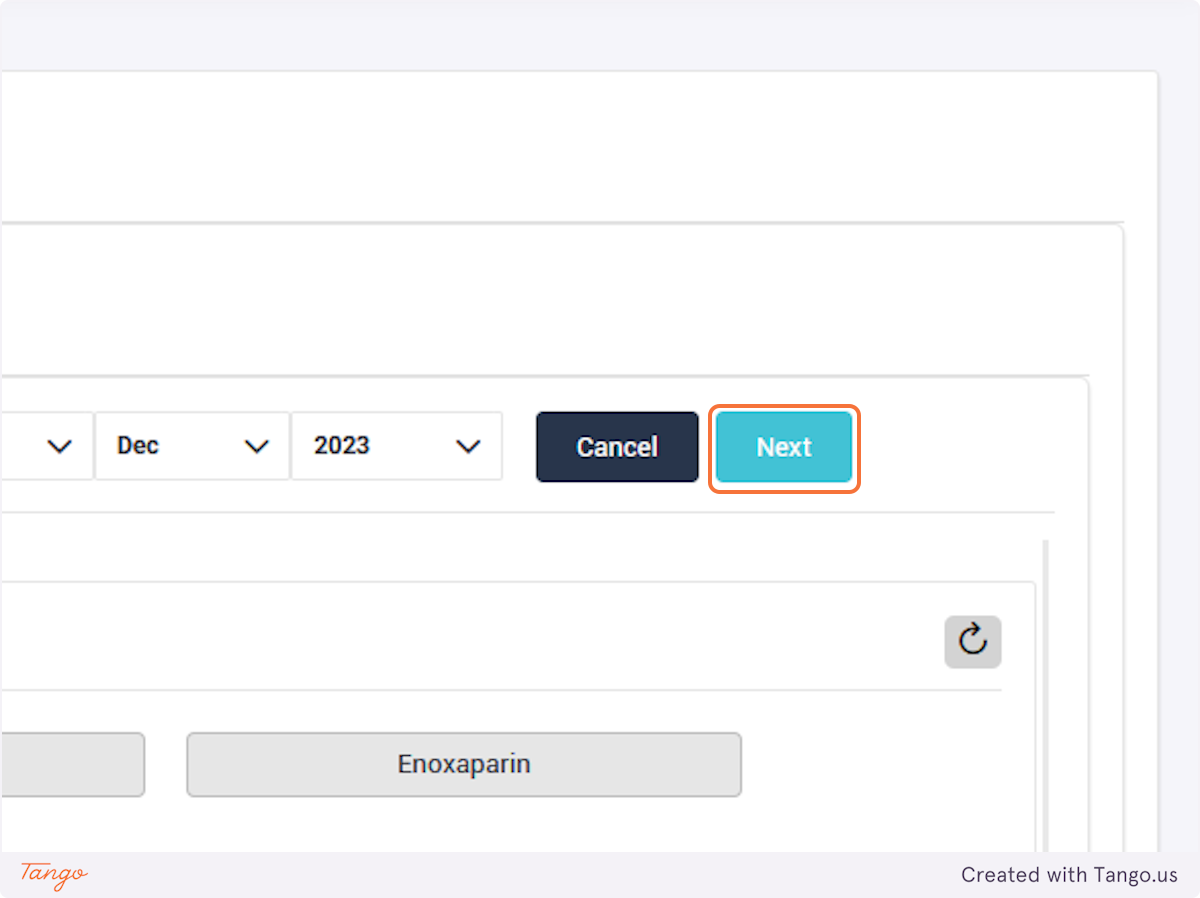
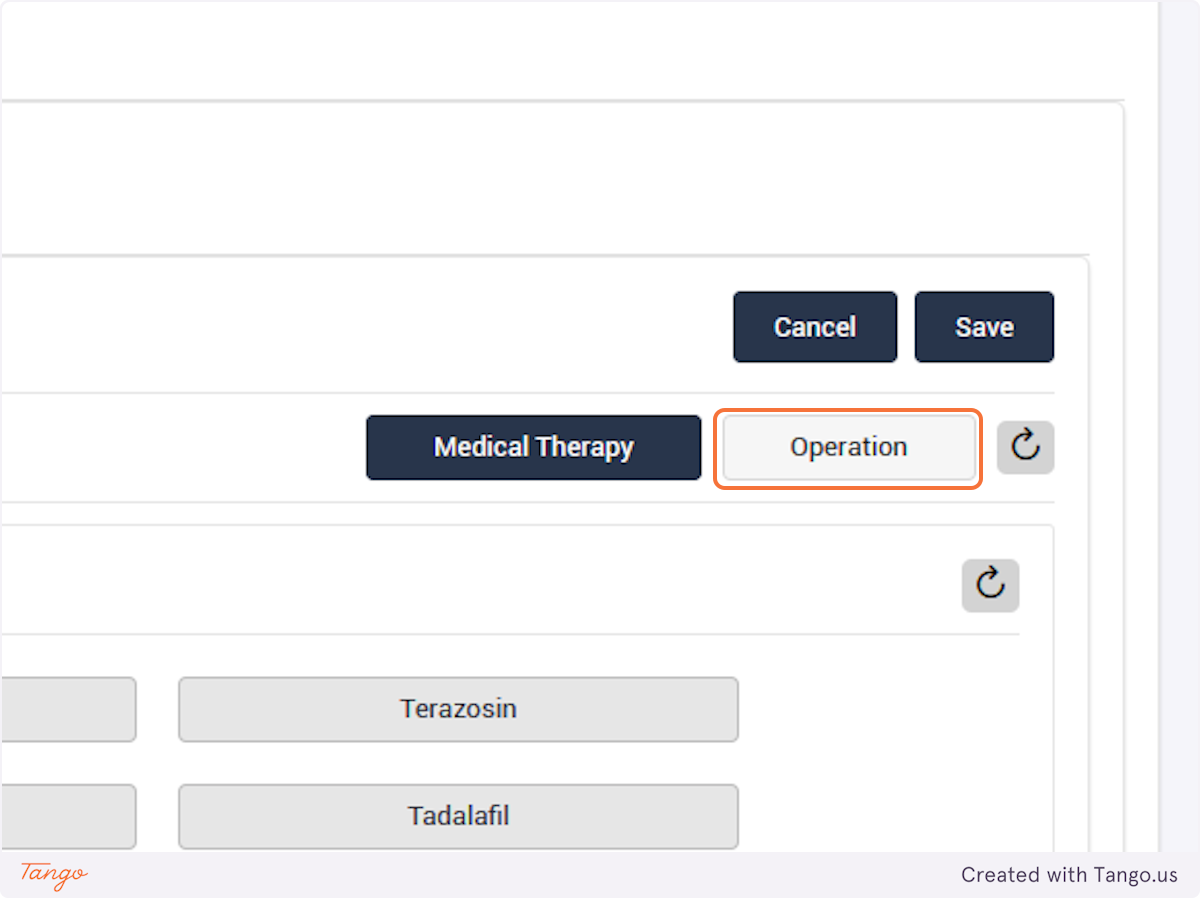
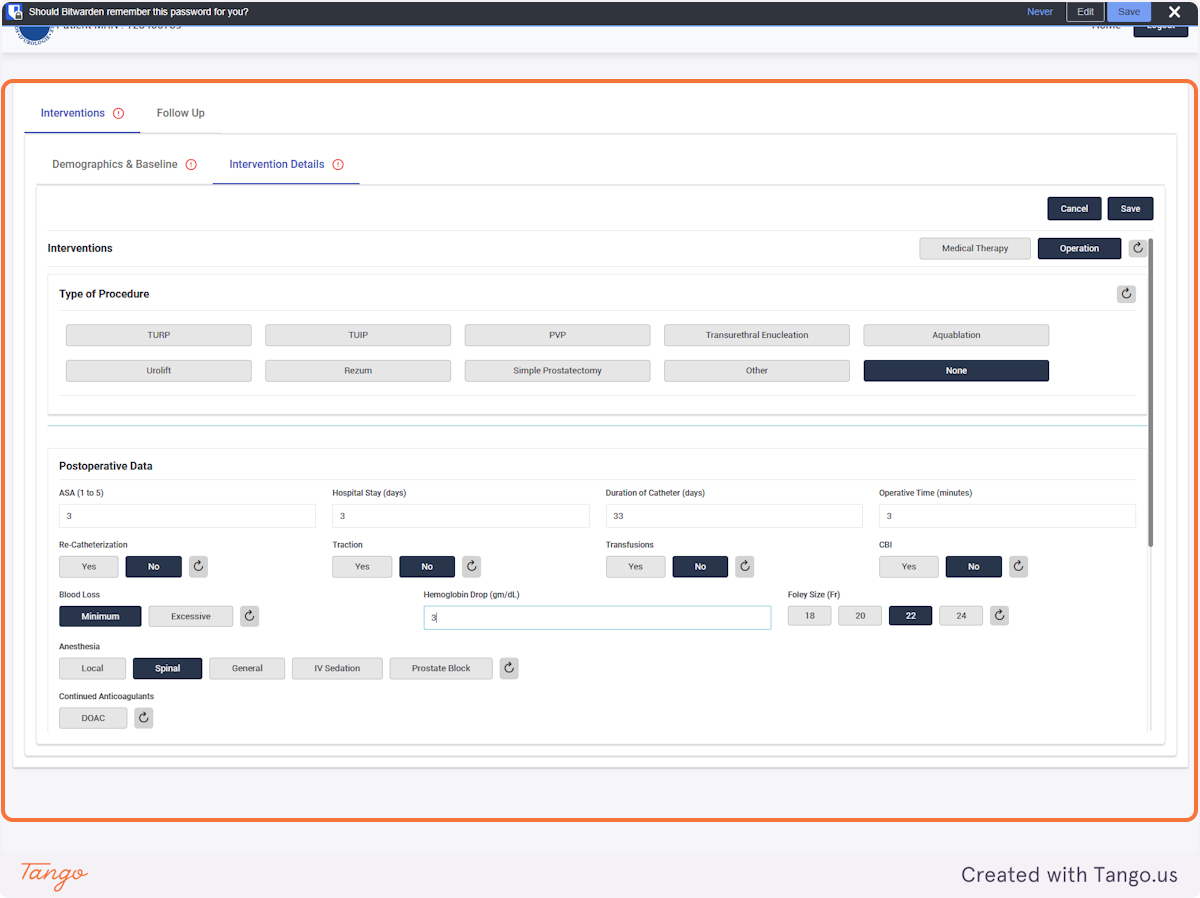
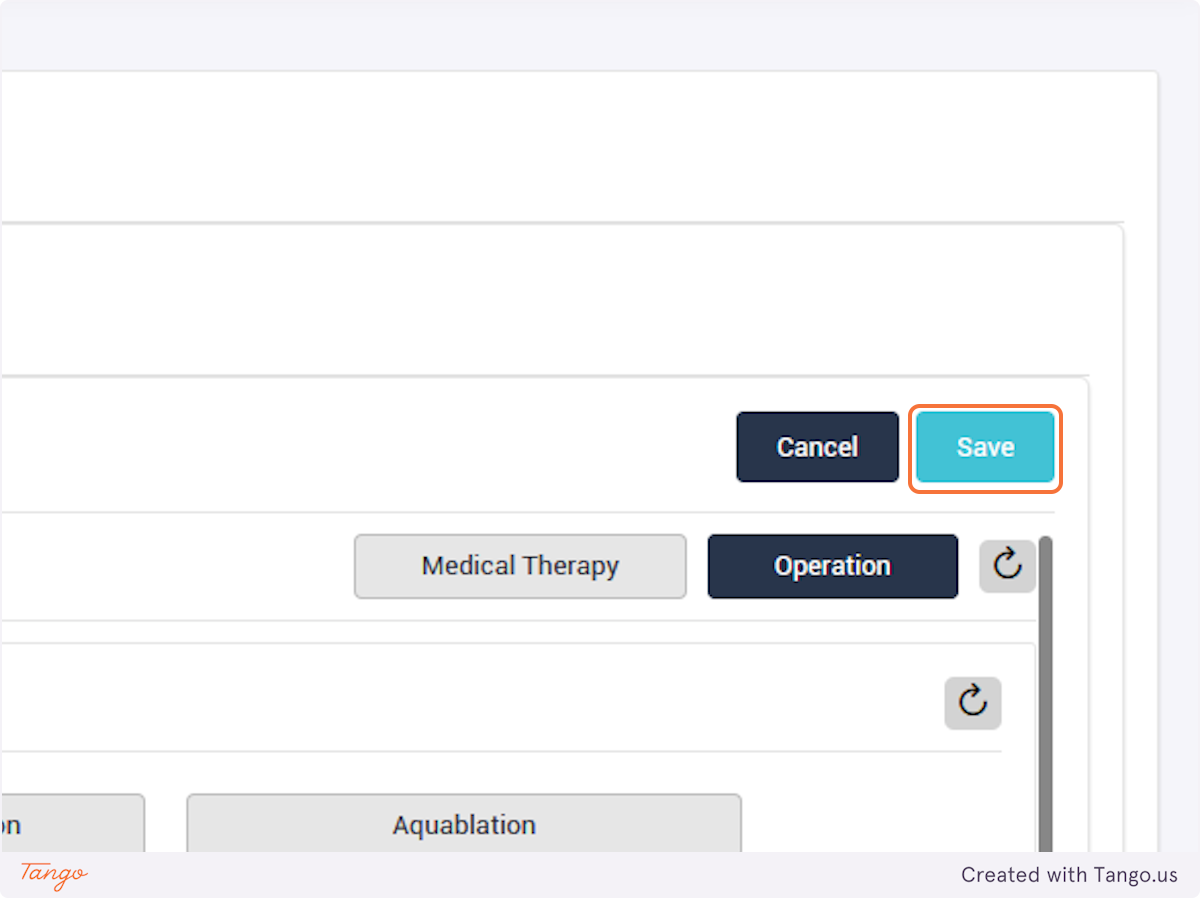
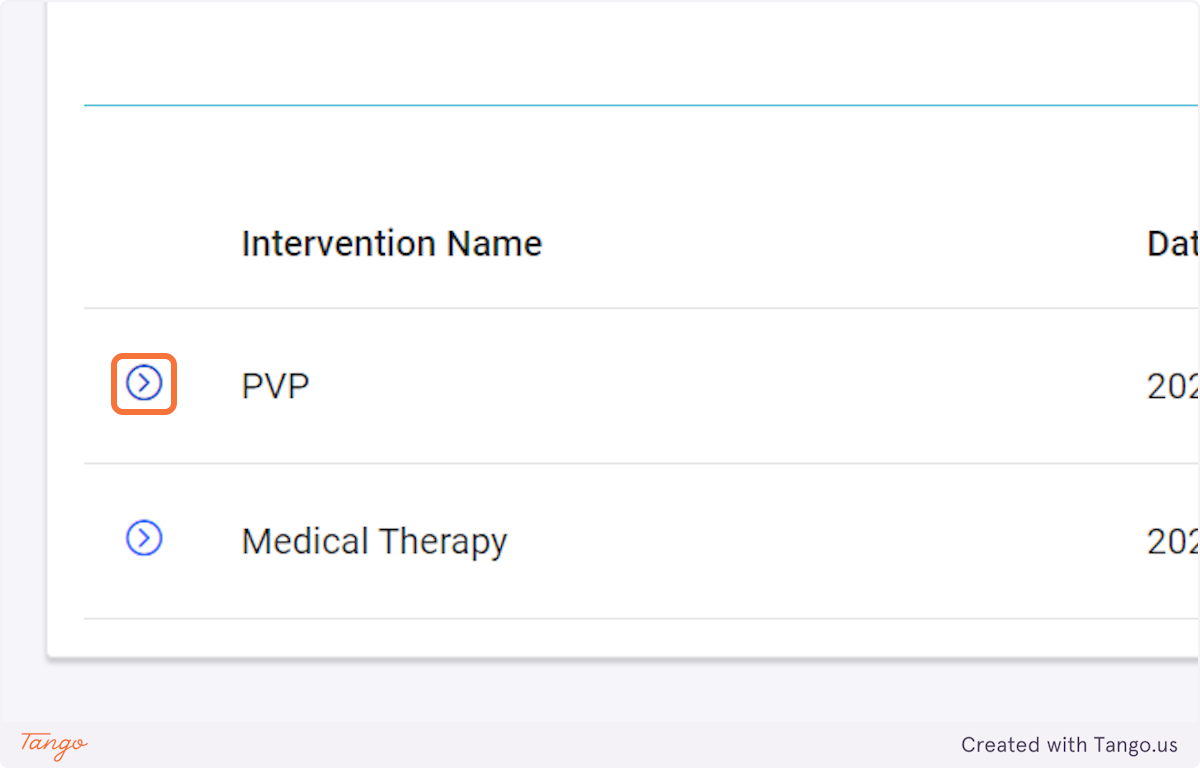
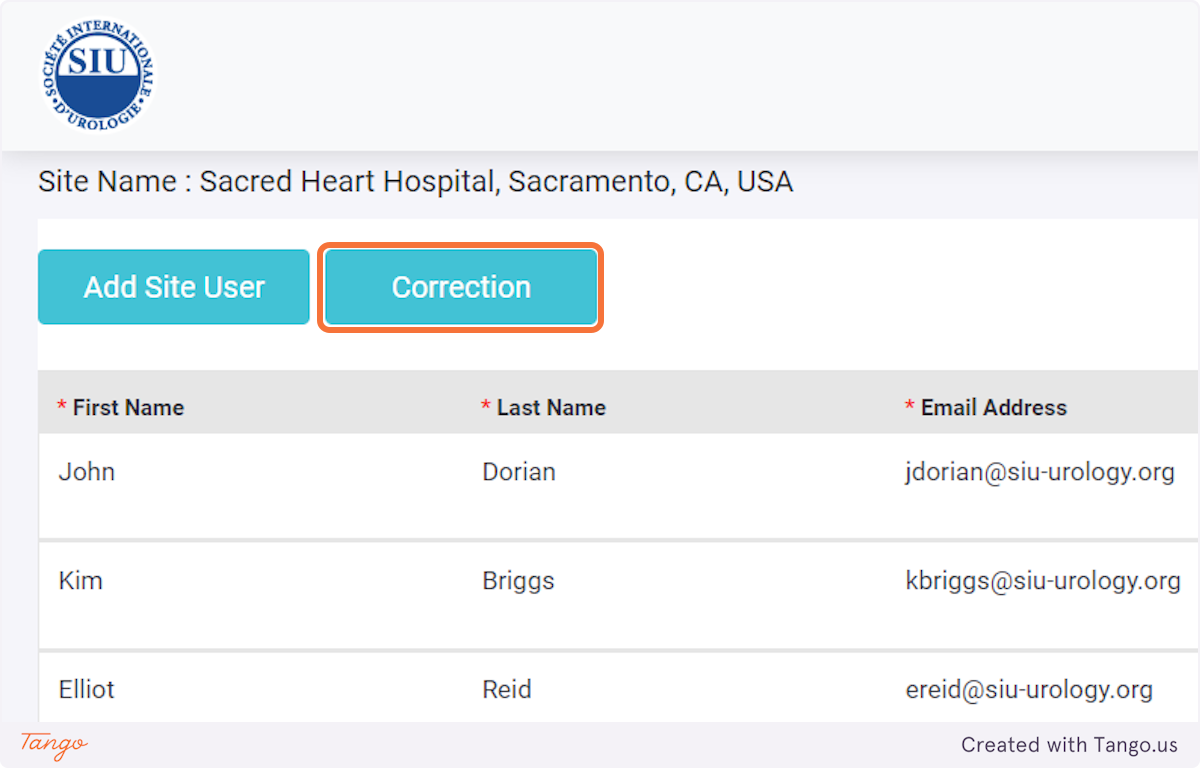
If an intervention update is necessary instead, you may click the Intervention tab to fill any data that was previously missing. Please note that you cannot make corrections with a user account, you must ask your admin or use your own admin account to make corrections.
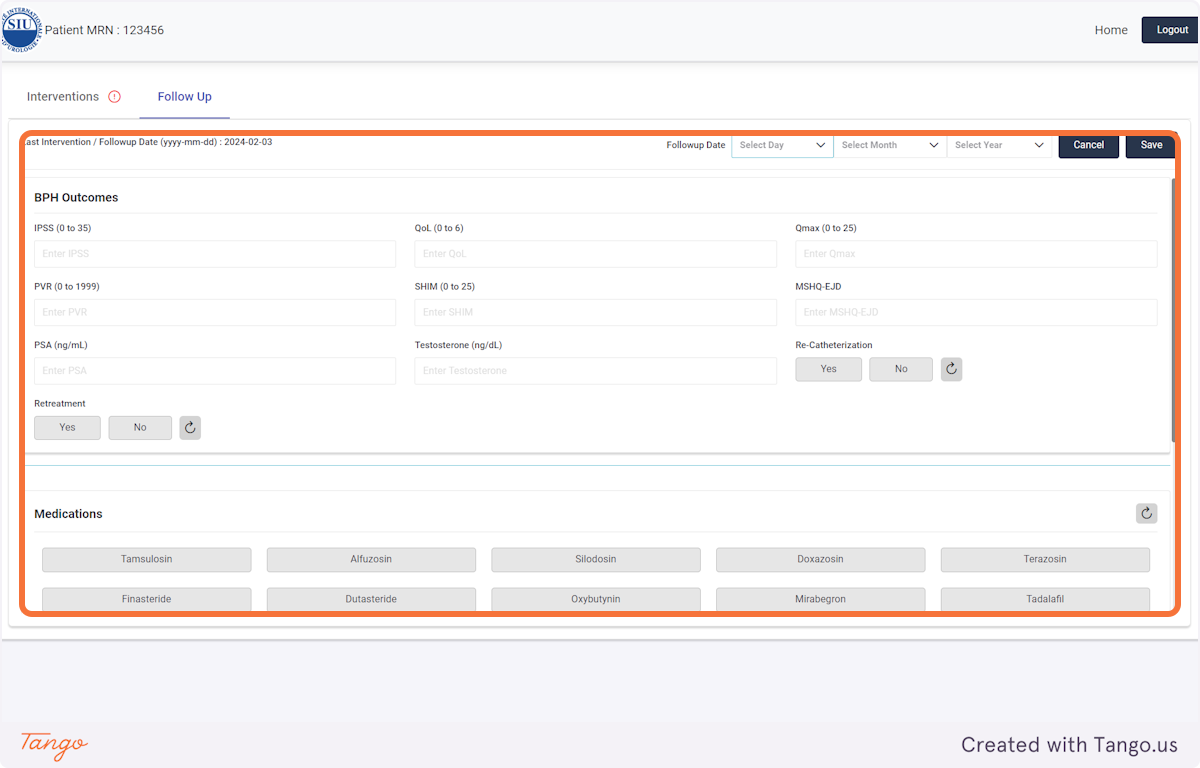
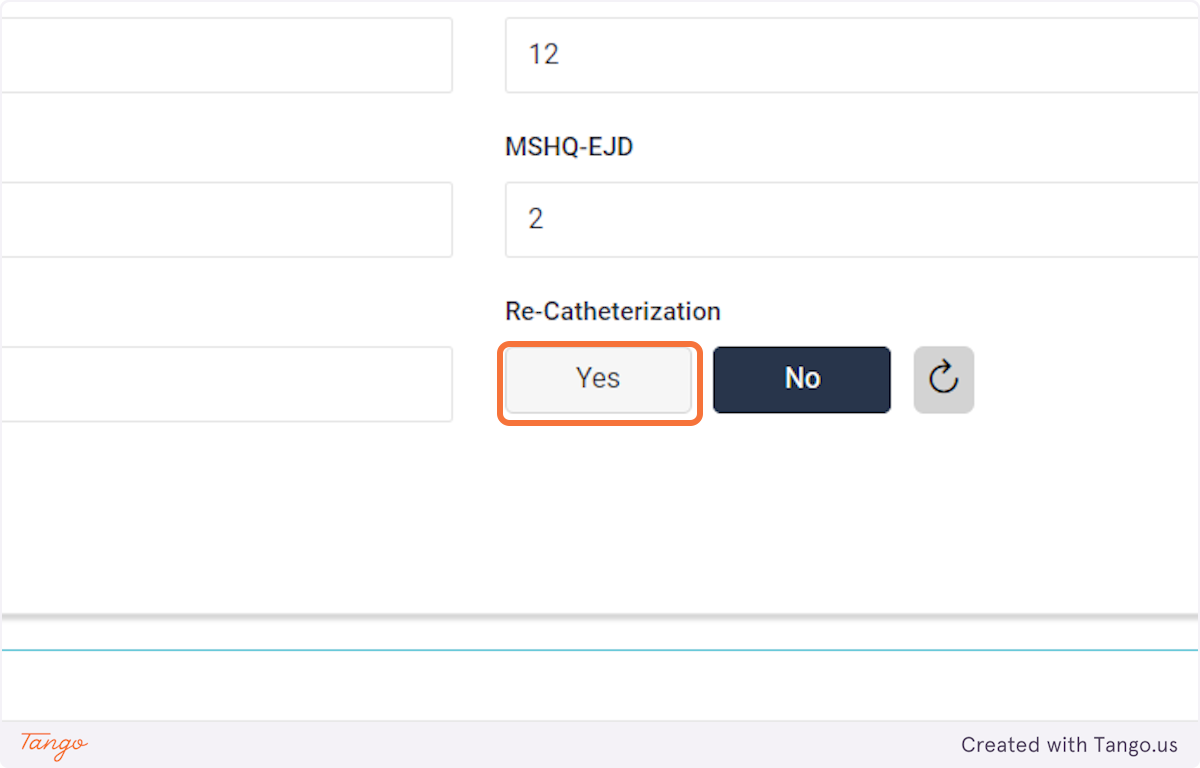
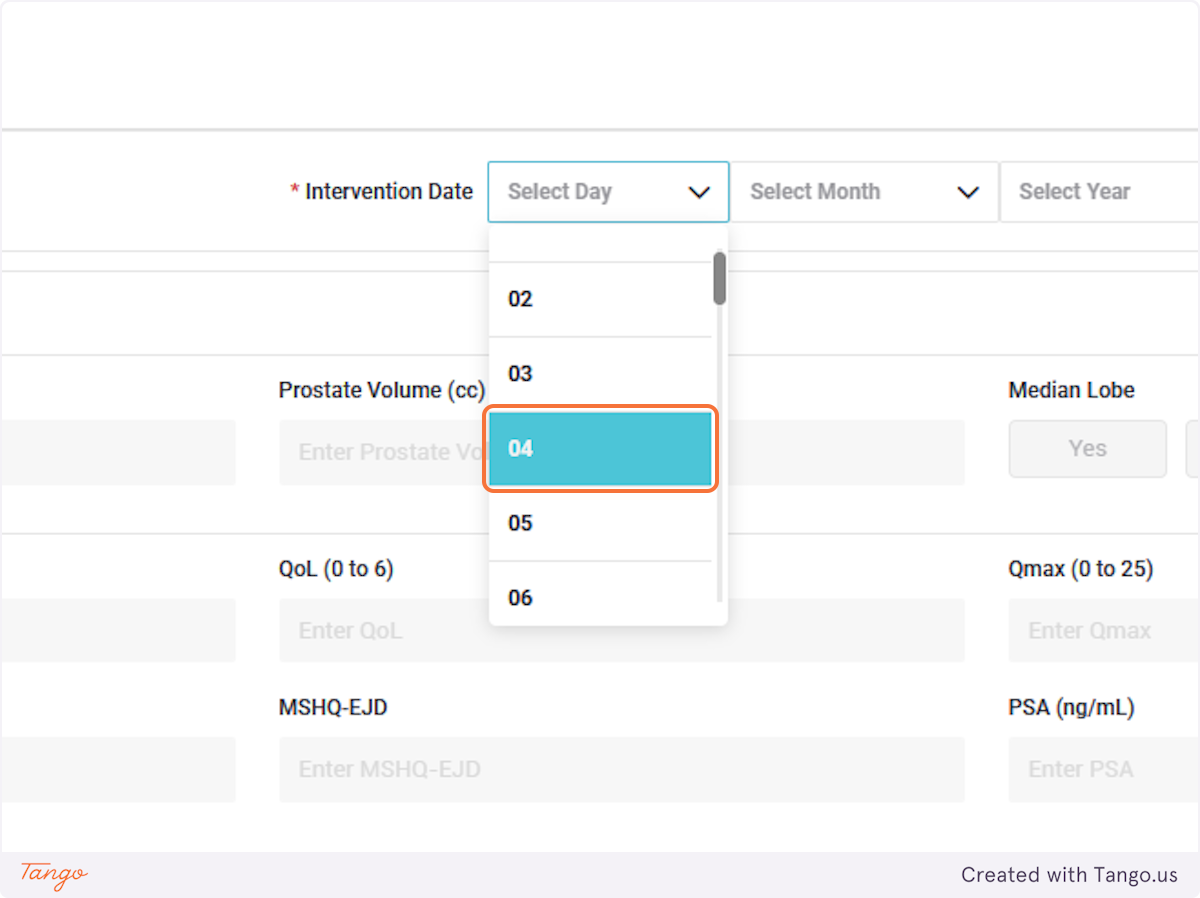
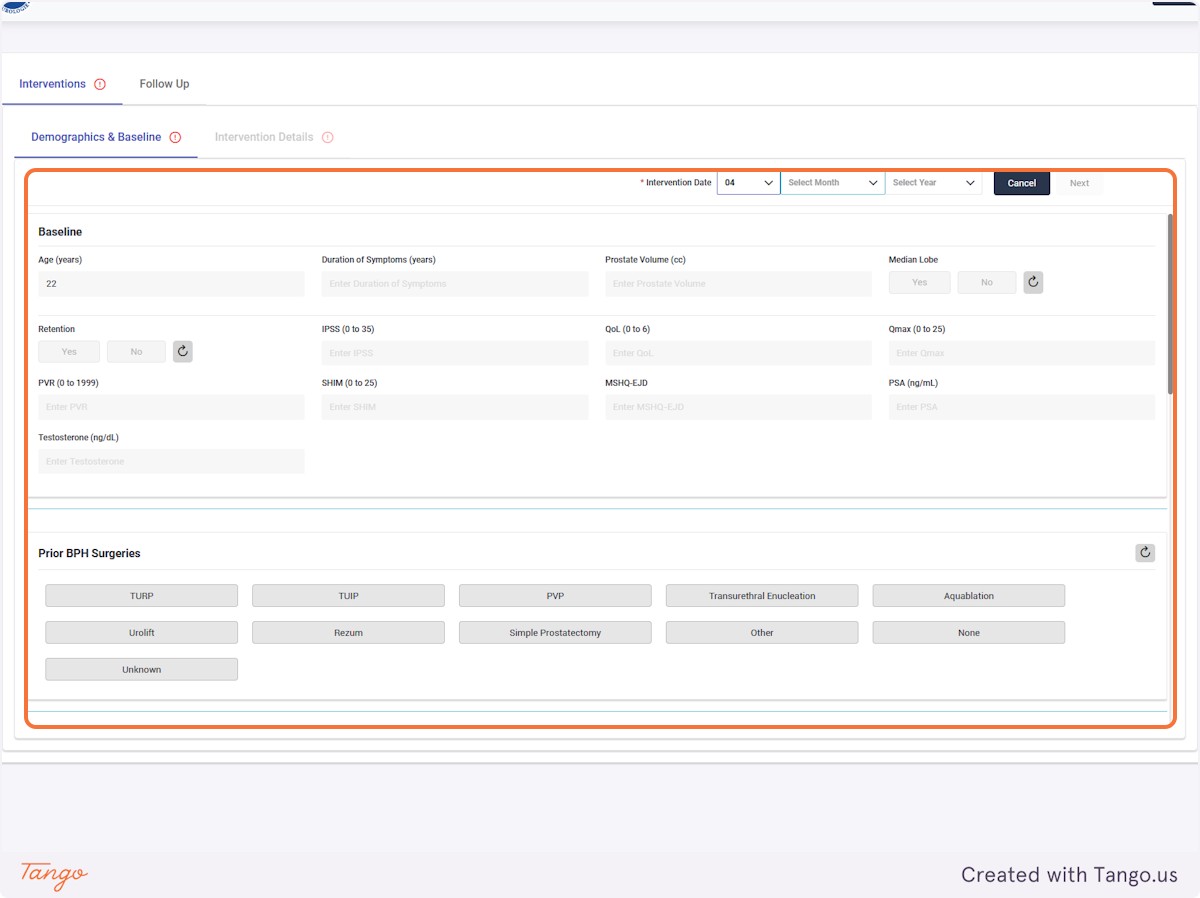
If you do not have certain variables recorded, they can be left blank and updated at a later date.
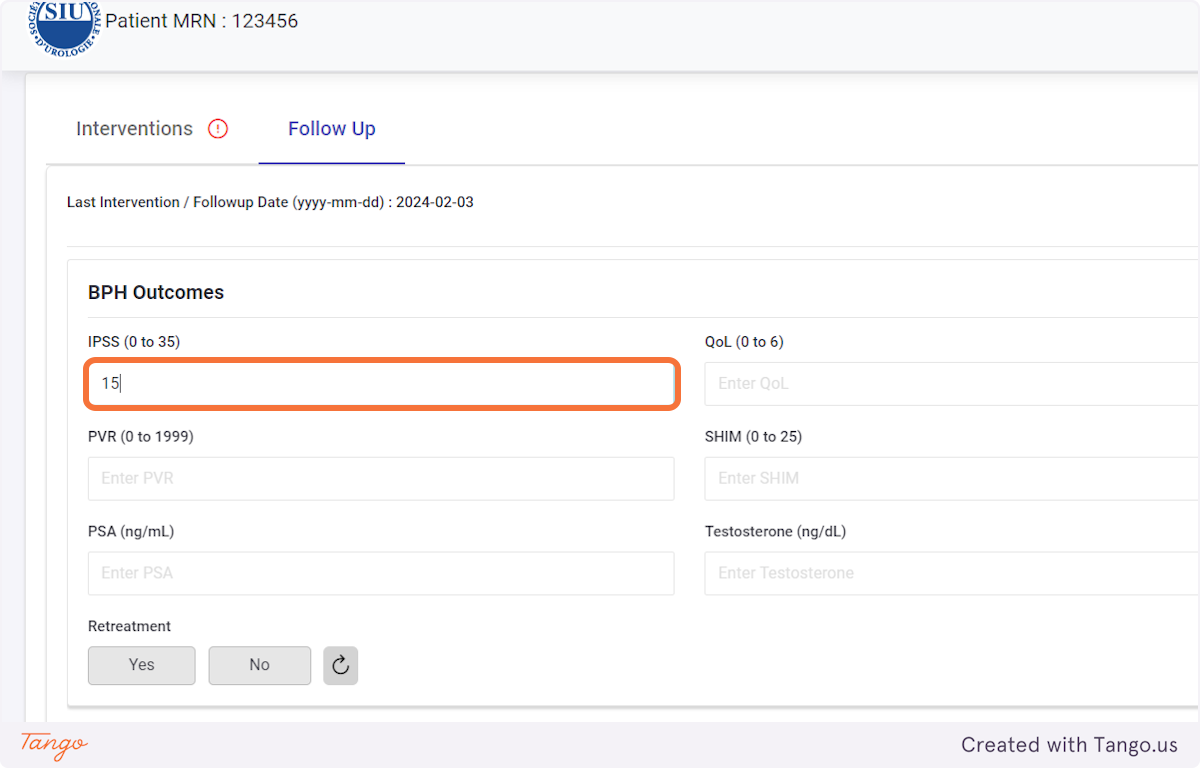
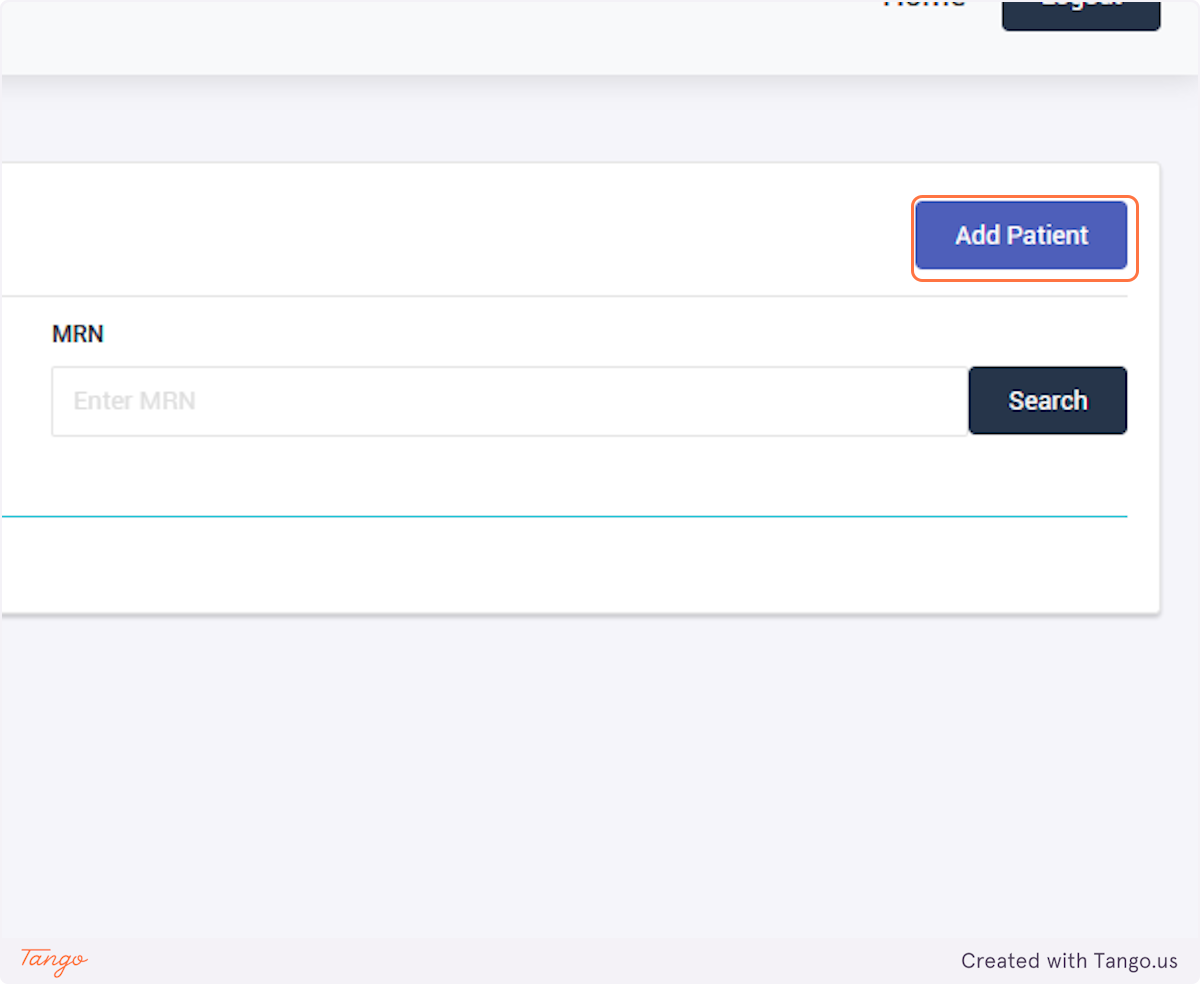
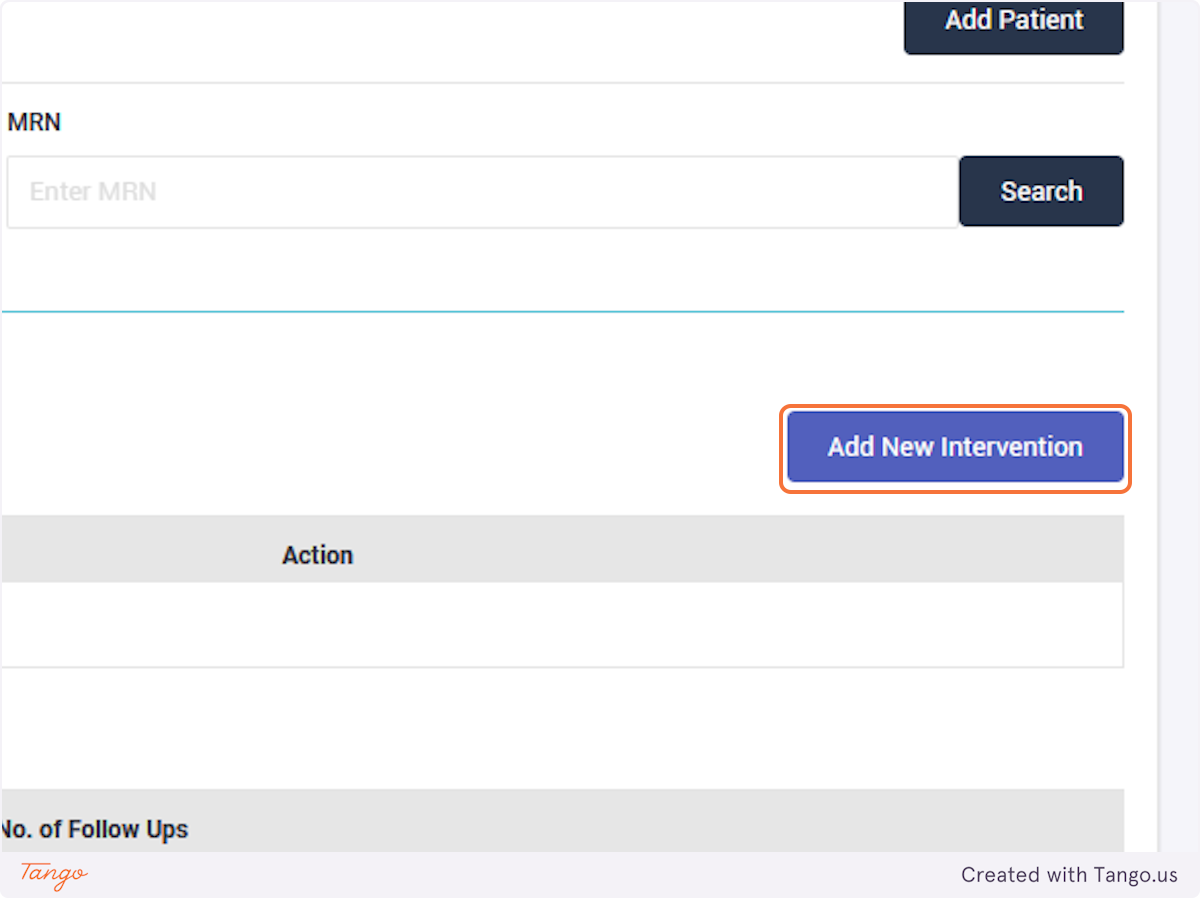
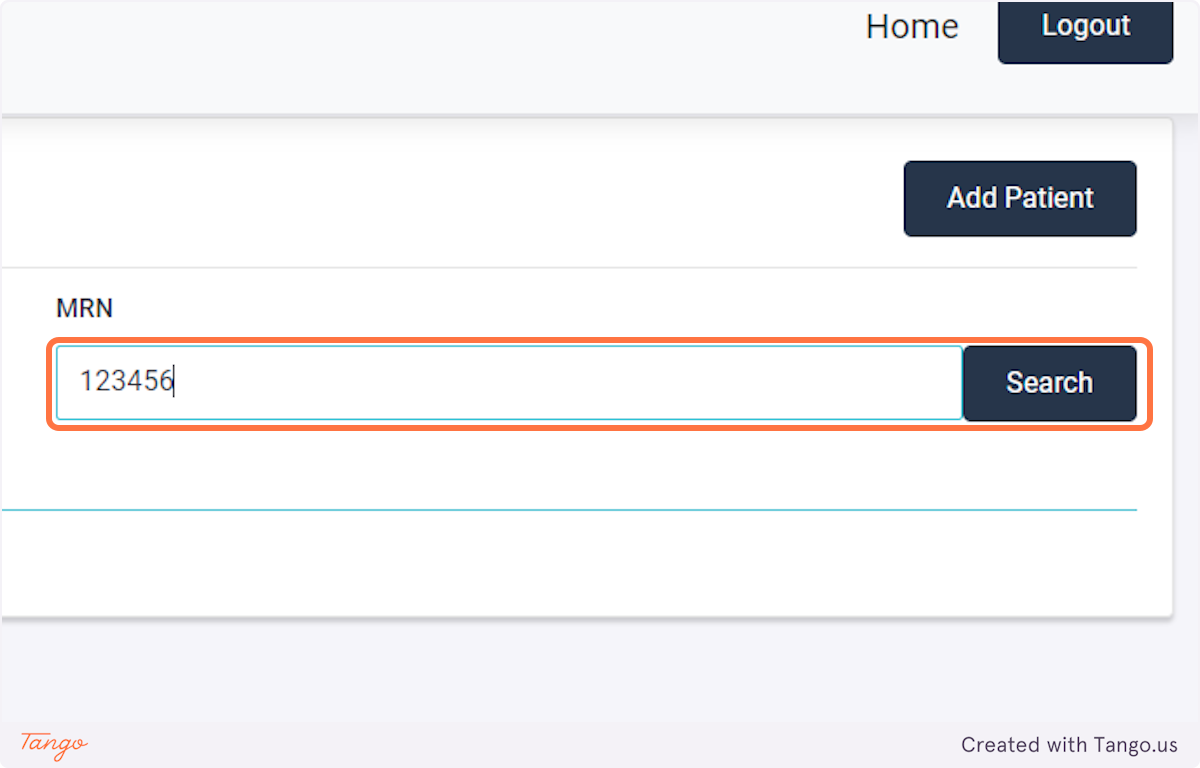
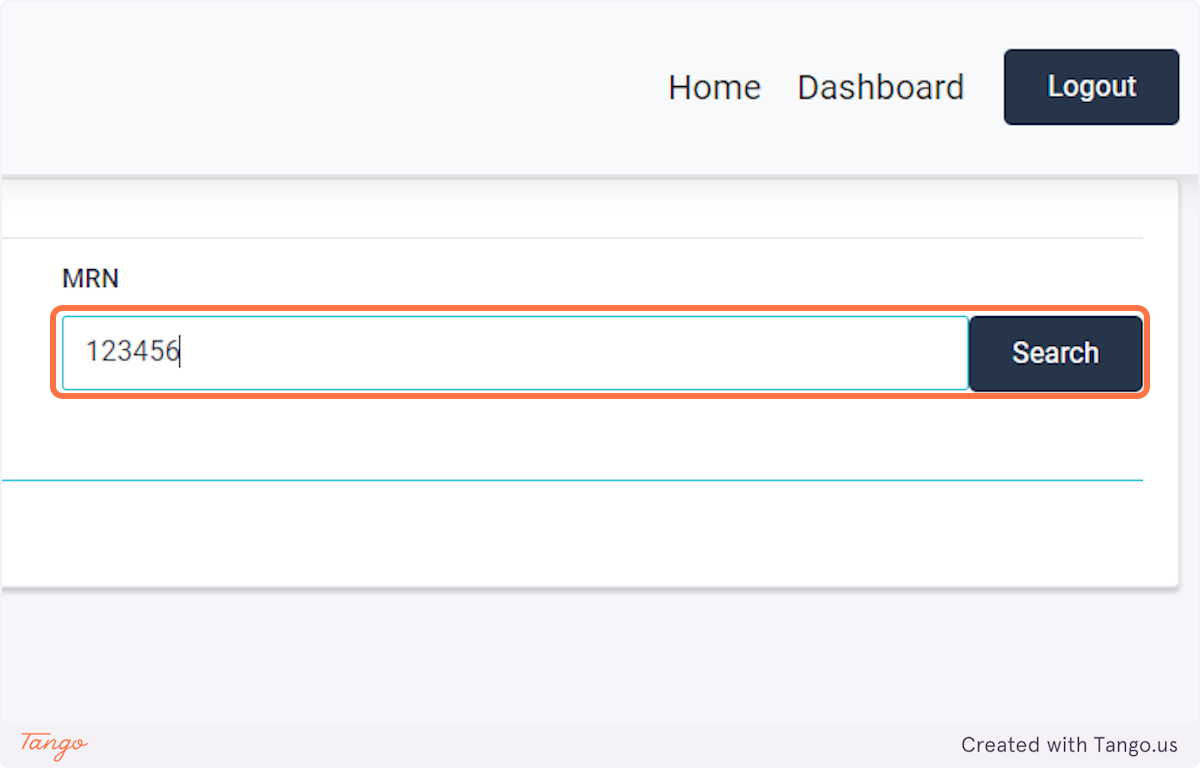
The registry will then take you to the home screen for this patient. If another patient's needs to be added next, you may search for them using the MRN searchbox again.
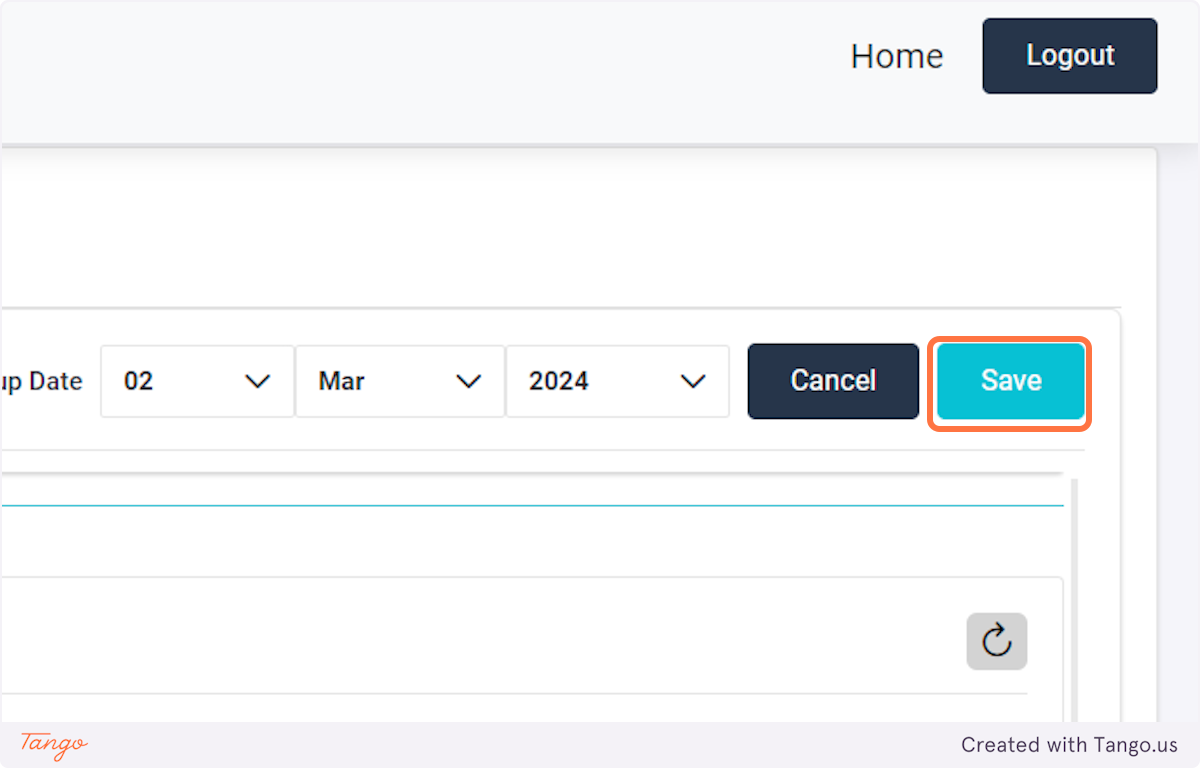
Note that to add users, you first need to log in with an administrative account.
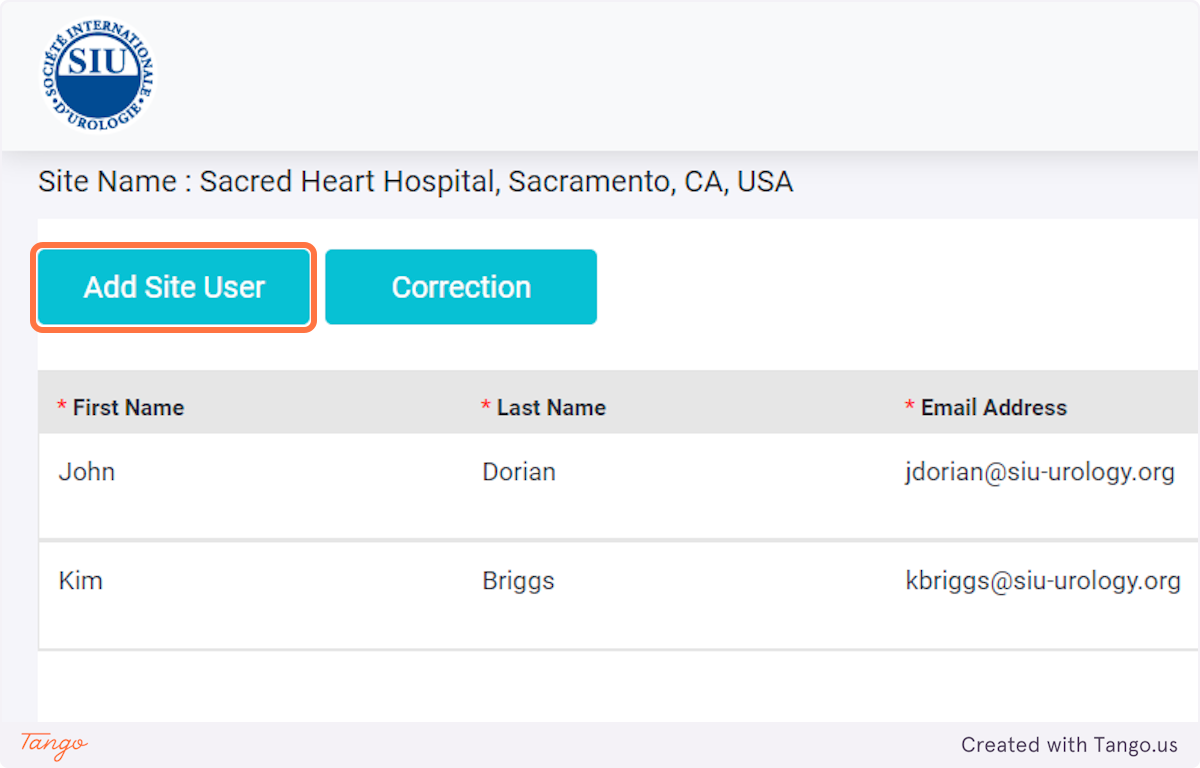
Tip: Use a password manager to generate a random password. Save this password in an email draft as you will need to share it with the user via email.
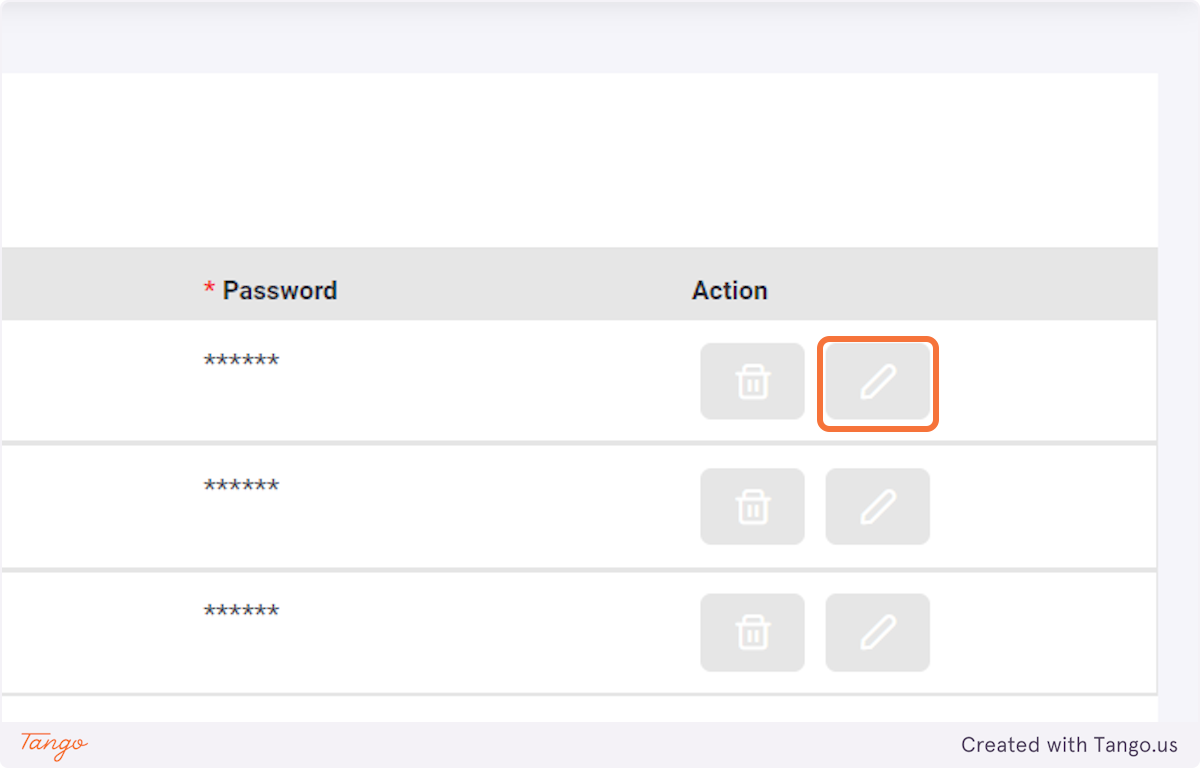
In case an error was made when creating the user, or the password needs to be changed, select the pen button and update the account.
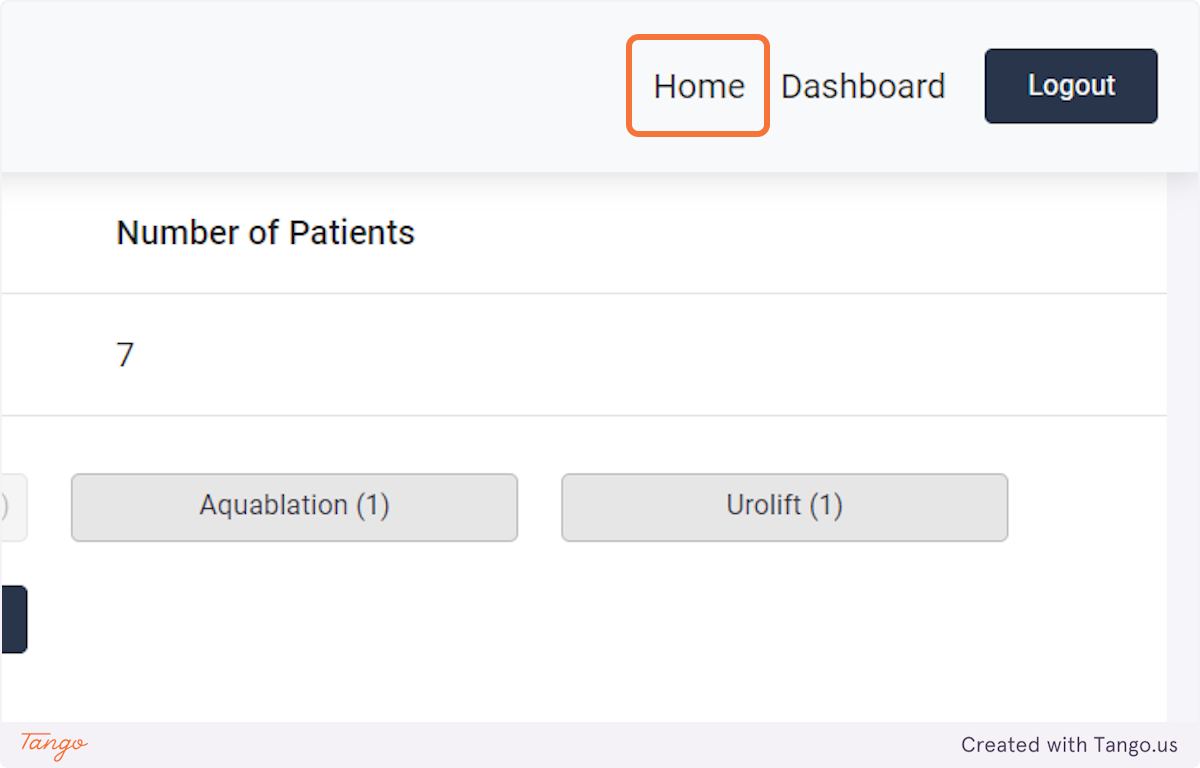
Note that you may use filters to narrow your patient population.
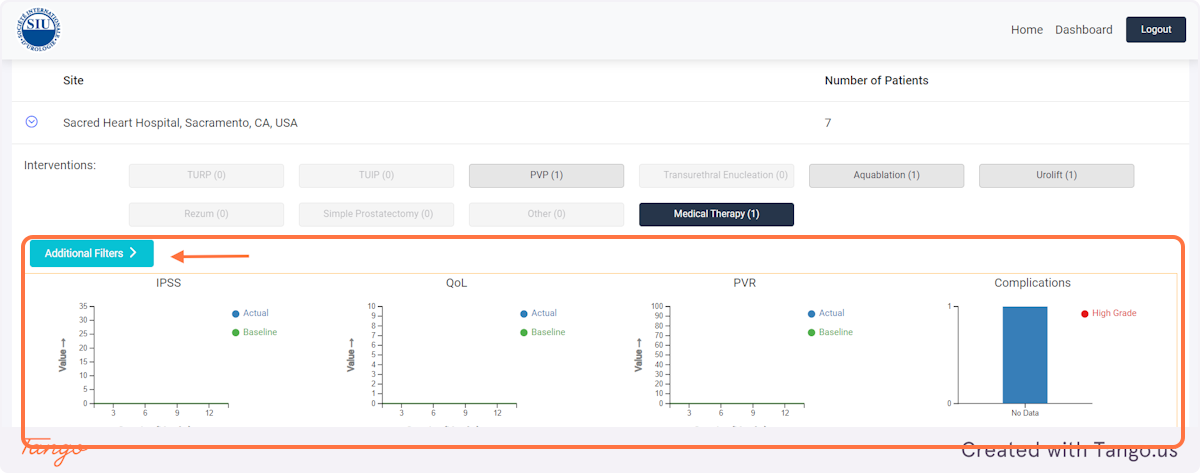
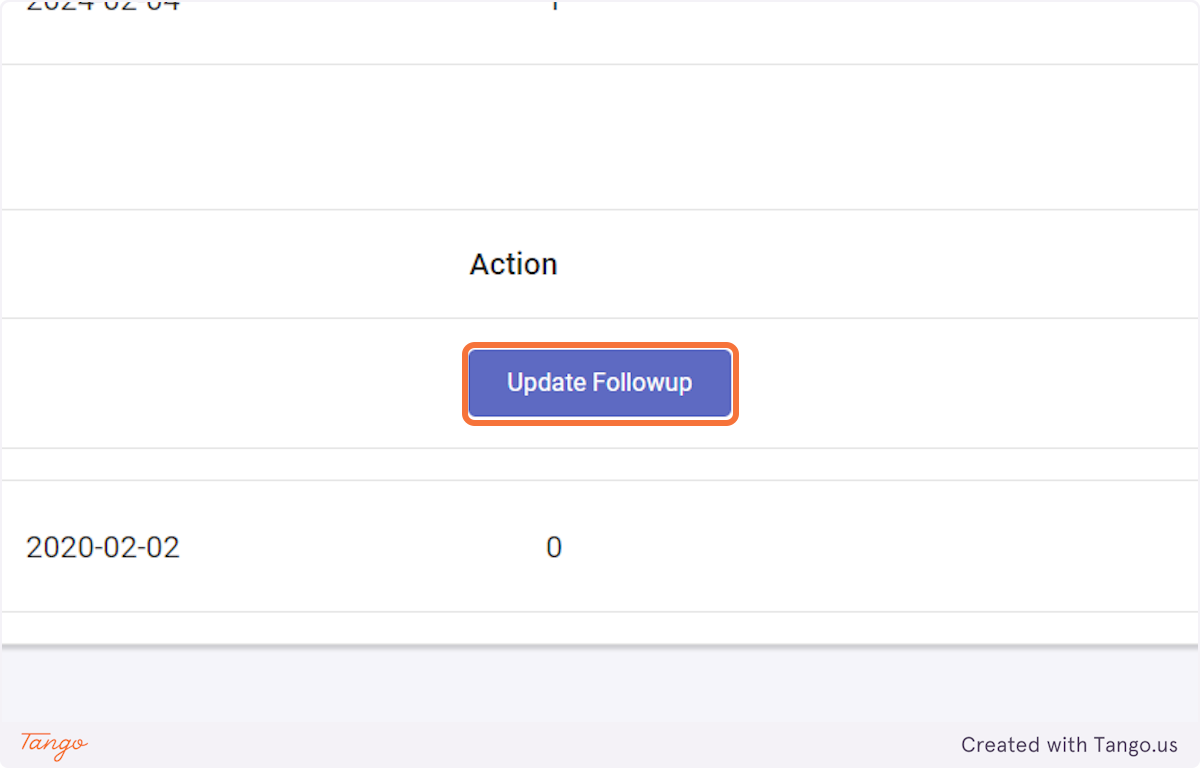
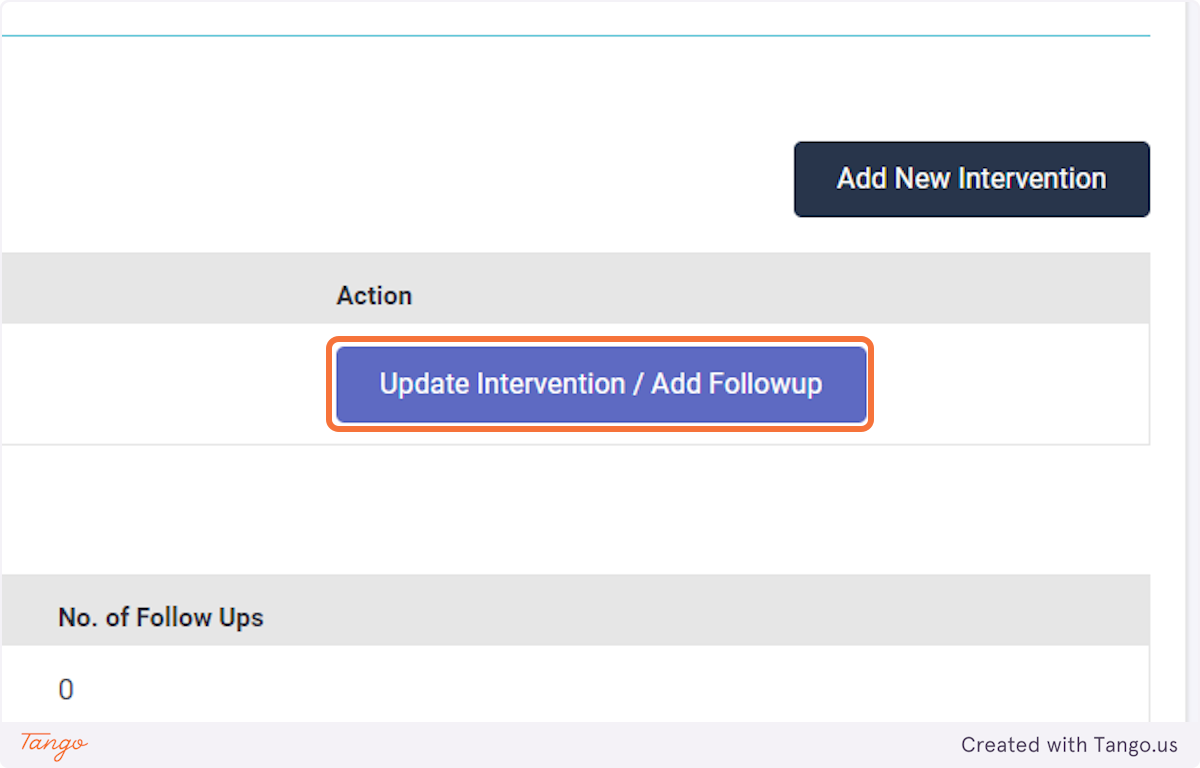
If you would like to correct an intervention rather than a followup then select "Update Intervention" instead.